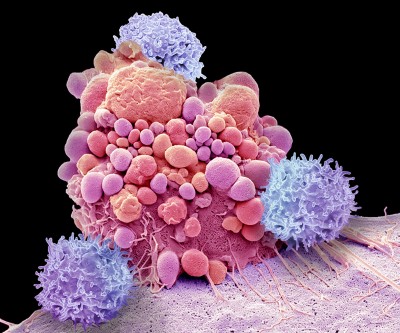
A brain tumour called a glioblastoma (orange; artificially coloured). Researchers are now trialling engineered immune cells against these deadly tumours and others called diffuse midline gliomas.Credit: Andre Labbe, ISM/Science Photo Library
Every two weeks at Seattle Children’s Hospital in Washington, a five-year-old child stops by for a fresh dose of genetically engineered immune cells administered directly into the fluid around their brain.
The child has been making these visits for more than three years, after they were diagnosed with a devastating form of brain and spinal cancer called diffuse midline glioma that has no known cure. But the treatment, called CAR-T-cell therapy, appears to have shrunk their tumour and kept it in check. At 70 treatments and counting, this five-year-old might have received more doses of CAR-T-cell therapy than anyone else on the planet.
His oncologist, Nicholas Vitanza, lights up whenever he talks about the results. Still, Vitanza is keenly aware that the child’s response is unusual. Although several children in Vitanza’s clinical trial might also have benefited from the CAR-T-cell regimen, most responses were not as dramatic or long-lasting as the five-year-old’s. Now, the question that keeps Vitanza and others in his field up at night is: how can they make that success less of an outlier?
At the International Symposium on Pediatric Neuro-Oncology in Philadelphia, Pennsylvania, which ended earlier this month, Vitanza and other researchers presented tantalizing early clinical-trial results that suggested CAR T cells could be effective treatments for deadly central-nervous-system cancers in children.
The trials were designed to test the therapy’s safety rather than its effectiveness, and larger trials are needed to know for sure whether the treatments are beneficial. In the meantime, researchers are eager to find ways to tweak their approach to maximize its reach. “We’re seeing a glimpse of a signal” that the approach could work, says Jasia Mahdi, a paediatric neurologist at Texas Children’s Hospital in Houston. “Our task now is to figure out how we expand on that.”
Tumour-finding T cells
CAR-T-cell therapies consist of immune cells called T cells that have been removed from the recipient and engineered to produce molecules dubbed chimeric antigen receptors (CAR) on their surfaces. These T cells are readministered into the body, where their new receptors enable them to recognize and destroy cancer cells.
Last-resort cancer therapy holds back disease for more than a decade
Despite lingering safety concerns, the approach has shown success in treating several blood cancers and, in some cases, has produced remissions lasting more than a decade. But using CAR-T-cell treatments to treat solid tumours such as those of the brain and lung is more challenging. Solid tumours can contain various cells with different mutations and differing sensitivities to the therapy. Solid tumours can also be more difficult for the T cells to penetrate.
Even so, studies in mice have suggested that CAR T cells might work against diffuse midline gliomas. New therapies for the cancer are desperately needed: the standard treatment is radiation occasionally paired with chemotherapy, but the cancer is fatal and median survival is about 13 months after diagnosis, says Vitanza.
Success: a diploma
Now, the first CAR-T therapy clinical trials against diffuse midline gliomas in children have finished, and the results are promising. At the meeting in Philadelphia, Vitanza presented data from a trial in which 21 children with diffuse midline glioma were treated with CAR T cells that target a protein called B7-H3, which is found predominantly on cancer cells. Only one of those participants experienced a severe reaction to the treatment itself, and some have lived longer than expected, Vitanza says.
Mahdi presented data from a clinical trial of a T-cell therapy that targets a molecule called GD2. In that trial, conducted at Stanford University in California, nine people with diffuse midline glioma received treatment, and tumours shrank by more than half in four of them.
That trial also had an outlier: a young man whose cancer disappeared entirely and who has remained cancer-free for the more than 30 months since his first treatment. In that time, he has graduated high school and is now thriving at university. “All those normal things mean so much more in this context,” says Mahdi. “This reality wouldn’t have been his otherwise.”
A menu of choices
The researchers are eager to find ways to extend these dramatic responses to more of their study participants. Vitanza’s team has launched another trial that will test CAR T cells that target four different molecules found predominantly on brain and spinal tumours, in hopes that T cells that recognize multiple targets will be more effective.
Searching for the roots of brain cancer
Another team at the University of California, San Francisco, is testing CAR T cells that express the cancer-seeking receptor only when the cells are in the central nervous system. The hope is that the T cells will be active solely where they are needed, making them less likely to become dysfunctional from “exhaustion”, a phenomenon known to limit the effectiveness of T-cell therapies, says Hideho Okada, who studies immunotherapies and is a lead investigator on the project. The team treated their first clinical-trial participant — an adult with an aggressive brain cancer called glioblastoma — in June and plans to launch a similar study in children next.
Such modifications to CAR-T-cell therapies are only the beginning, says Vitanza. Researchers are hunting for more ways to supercharge CAR-T-cell therapies, and decades from now, physicians might be able to pick and choose from a variety of options that can be tailored for individual patients. “It’s so incredible that we’ve gotten to this point,” he says. “But in 20 years, the CAR T cells we use for patients will look very different from now.”