
Craig Ramirez and Andrew Hauser used to brainstorm ideas about their research over beers.Credit: Ariana Drehsler for Springer Nature
Cancer likes a drink — and so do Craig Ramirez and Andrew Hauser.
As members of Dafna Bar-Sagi’s cancer-biology laboratory at the New York University (NYU) Grossman School of Medicine, Ramirez and Hauser had been studying the molecular complexities of a process exploited by tumours to fuel their unremitting growth. Known as macropinocytosis, the mechanism enables cancer cells to ‘drink’ surrounding fluids, thereby slurping up vast quantities of protein and fat to support their metabolic needs.
By 2017, the two researchers — Ramirez with his cell-biology expertise, and Hauser with his biochemical acumen — had unravelled the mechanism by which tumour cells use macropinocytosis to satisfy their nutritional demands. They focused, in particular, on mutations in the RAS protein, a crucial player in the genesis of about one-third of all cancers. But they were not quite ready to publish their results — a series of validation experiments remained.
After official work hours, Ramirez and Hauser would move to The Albion, a now-defunct bar in the Kips Bay neighbourhood of New York. There, over pints of craft beer, the duo would brainstorm ways to convert their scientific discoveries into tangible treatments, aiming to improve care for individuals grappling with RAS-driven cancers.
Turning the tables on cancer
The most straightforward approach, they thought, would be to develop drugs that target the macropinocytosis pathway and cut off a vital survival route for RAS-mutant tumour cells. But cognizant of cancer’s ability to develop resistance against such inhibitory strategies, the researchers considered a more innovative tactic — one that would exploit, rather than simply obstruct, the tumour’s method of nutrient uptake.
Ramirez recalls thinking, over sips of IPA beer: “If these cancer cells are already consuming protein for survival and growth, instead of trying to cut off signalling why not just come up with a protein carrier and attach toxins to it like a Trojan horse?”
The researchers found a promising carrier in the laboratory of NYU protein engineer Shohei Koide, who decades earlier had invented a synthetic protein framework called a monobody. Experiments revealed that RAS-mutant cancer cells readily absorbed these monobodies through macropinocytosis1.
Teaming up with Nathan Beals, a nanoscientist and fellow member of the Bar-Sagi research group, Ramirez and Hauser designed a drug candidate that linked Koide’s monobody to an anticancer compound — a toxin derived from a defensive peptide originally discovered in a marine mollusc. The resulting construct, termed a protein–drug conjugate, allowed for administration of “really toxic payloads at much higher doses” than those that other delivery technologies can safely handle, says Beals (see ‘Drinking in the therapy’).
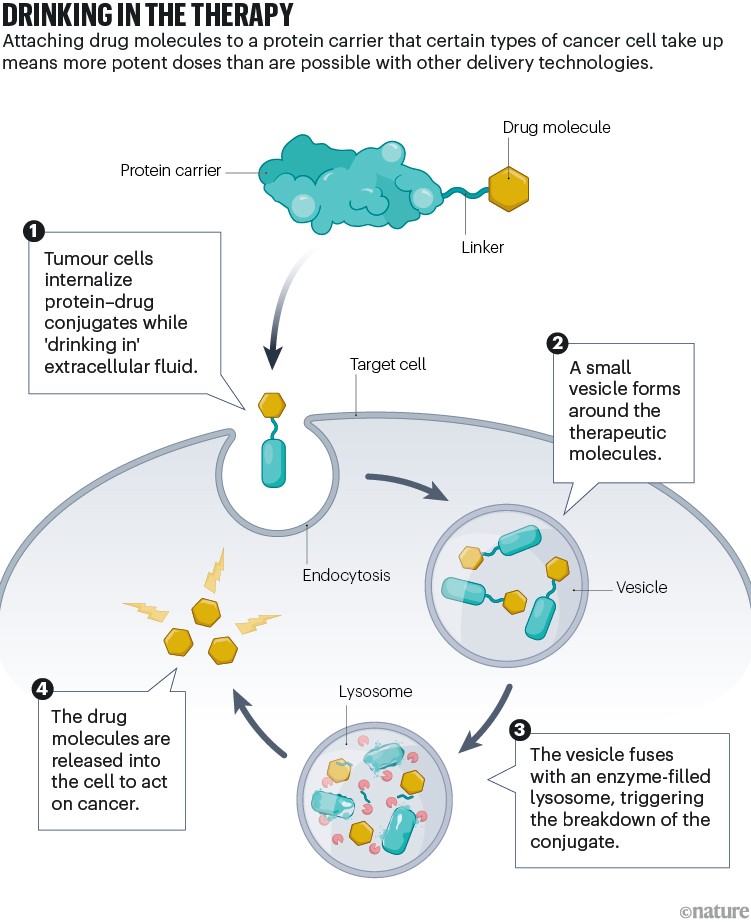
Infographic by Alisdair Macdonald
Ramirez and Hauser now had their technology platform. They secured the necessary intellectual property rights from NYU and formed a company in 2019. Later that year, they reported their findings on the use of macropinocytosis against RAS-mutant cancers2. In a nod to the start-up firm’s pub beginnings, they named their company Tezcat — after Tēzcatzontēcatl, the god of drunkenness in Aztec mythology. Ramirez took the helm as chief executive and Hauser serves as chief operating officer.
Concentrated effort
Since its inception, Tezcat — which maintains a small lab space in New York City and offices in New Haven, Connecticut — has pulled in millions of dollars in research funding from the US government and from competitions backed by the pharmaceutical industry. The money has allowed the company to test its lead drug candidate, called TZT-102, in mouse models of RAS-altered pancreatic cancer, lung cancer and multiple myeloma.
“Some of the results we have are quite staggering,” says Beals, who is a consultant for Tezcat and helped to carry out these experiments. Treatment with TZT-102 prompted every type of cancer to shrink in the mice, outperforming standard-of-care treatment options. What’s more, the therapy showed an array of favourable pharmacological properties, suggesting it could be both safer and more effective than other drug-delivery platforms.
“It gets concentrated at really good levels in the tumour cells,” says Gareth Morgan, a haematologist who directs the multiple myeloma research programme at NYU’s Perlmutter Cancer Center. Morgan and his haematologist colleague Faith Davies, director of the Center for Blood Cancers also at Perlmutter, collaborated with Tezcat on the myeloma study, along with Beals and Bar-Sagi3.
Scientists who study the role of macropinocytosis in RAS-driven cancers are optimistic about the technology’s implications for drug targeting. Feng Qian, a cancer therapeutics researcher at Tsinghua University in Beijing, describes the mouse data as “impressive, especially in terms of tumour inhibition efficacy”, with notably minimal side effects.
Tezcat is not alone in pursuing drug-delivery approaches that try to take advantage of micropinocytosis, but results elsewhere have often been disappointing. In some cases, the drugs end up being cleared away by immune cells. At other times, they gather in places such as the spleen and liver, and damage healthy tissues.
Tezcat’s approach of deploying monobodies that target RAS-linked vulnerabilities, but that are still cleared quickly from the body, might be particularly effective at promoting tumour accumulation and minimizing off-target effects, says Miles Miller, a cancer biologist in the Center for Systems Biology at the Massachusetts General Hospital, Boston. “Their initial results seem promising,” he says.
Expanding horizons
Tezcat is seeking further investment as it prepares to kick off clinical trials for TZT-102 in the coming two to three years.

Andrew Hauser and Craig Ramirez combined their expertise to come up with a drug-delivery platform.Credit: Alex Efron
The company’s technology is adaptable — the same macropinocytosis-prone monobody can be used to carry many different payloads, including radioactive isotopes, immune-activating agents and vaccine components. For example, a second drug candidate in Tezcat’s pipeline, called TZT-107, is crafted to administer a powerful stimulant of anti-cancer immunity.
Looking ahead, the Tezcat founders see potential in applying their technology to other maladies that are influenced by micropinocytosis, such as neurodegenerative disorders, inflammatory conditions and cardiovascular diseases.
The platform could also find purchase in the diagnostics arena, says Ramirez, particularly with its capacity to help provide real-time visualization of tumours during surgical procedures or monitoring disease progress on imaging scans. “We see all these other shots on goal that are promising,” he says.
To avoid spreading Tezcat’s limited resources too thin, however, Ramirez and Hauser opted to establish a distinct entity for diagnostic applications. With a separate management team now in place, this venture — called IllumaRAS and based in Houston, Texas — aims to develop image-based tools that make use of the same monobody–conjugate design to deliver fluorescent dyes or radioactive isotopes to RAS-driven cancers.
Ultimately, the diagnostic and therapeutic applications of the technology could complement each other to enable precision-guided monitoring and treatment of RAS-mutated tumours. “Tezcat has the therapeutic approach, we have the imaging approach, and we will eventually converge at some point,” says IllumaRAS chief executive Enrique Gomez.
According to Ramirez, Tezcat’s progression from casual pub discussions to a distinctive drug-delivery platform underscores a successful blend of informal brainstorming and rigorous scientific research that many university spin-offs share. And the company’s journey highlights the transformative potential that can be unlocked when academics extend their laboratory discoveries into real-world applications, he adds.
“That all culminated into what we are today,” Ramirez says.
